
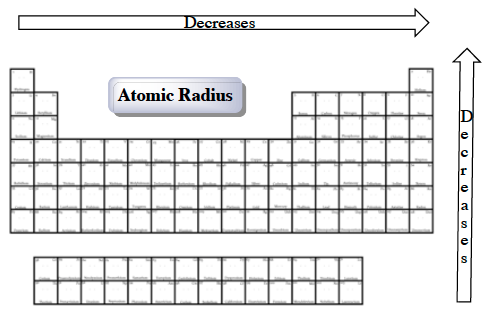
(2) Number of energy levels: The greater the number of energy levels, the larger the atomic radii. (1) Nuclear charge (number of protons) : The stronger the ‘pull’ the protons have to the electrons with electrostatic attraction, then the smaller the size of the atom radii Assuming atoms have a spherical shape, the radius of the sphere describes the size of the atom.Atomic Radii is affected by two main factors : Group: A column of elements in the periodic table of the chemical elements.Ītomic radius: A measure of the size of an atom. Period: A horizontal row in the periodic table, which signifies the total number of electron shells in an element’s atom.

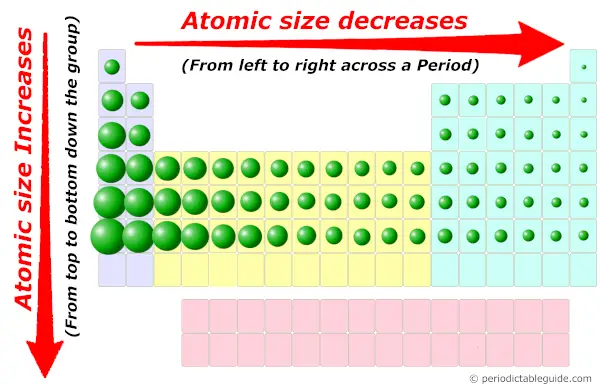
Atomic radii vary predictably across the periodic table.Sample Test C/P Section Passage 2 Question 7 Therefore, atomic size, or radius, increases as one moves down a group in the periodic table.Īpplications of Hard-Soft Acid-Base theoryĬhemistry Question Pack Passage 6 Question 29

In a noble gas, the outermost level is completely filled therefore, the additional electron that the following alkali metal (Group I) possesses will go into the next principal energy level, accounting for the increase in the atomic radius. The principal energy levels hold electrons at increasing radii from the nucleus. Therefore, the size of atoms decreases as one moves across a period from left to right in the periodic table. no effect at all as the two opposing tendencies of electron repulsion and nuclear attraction balance each other out.Įxperiments have shown that the first case is what happens: the increase in nuclear charge overcomes the repulsion between the additional electrons in the valence level.a decrease in size because of the additional protons in the nucleus,.an increase in atomic size because of additional repulsions between electrons,.These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom.Īs the atomic number increases along each row of the periodic table, the additional electrons go into the same outermost principal energy level (also known as valence level). Radii generally decrease along each period (row) of the table from left to right and increase down each group (column). The atomic radius of a chemical element is a measure of the size of its atoms and represents the mean distance from the nucleus to the boundary of the surrounding cloud of electrons.Ītomic radii vary in a predictable manner across the periodic table.


 0 kommentar(er)
0 kommentar(er)
